T-dependent Antigen Activation
Immunogenicity (formation of anti-drug antibodies) is an important problem associated with the administration of biological therapeutics. An immune response to these agents is dependent upon T-lymphocytes that are activated by the presentation of protein antigens associated with dendritic antigen presenting cells. This process results in activated T-helper cells that then interact with naïve B-lymphocytes that go on to produce specific antibodies directed against antigens present on the protein therapeutic.
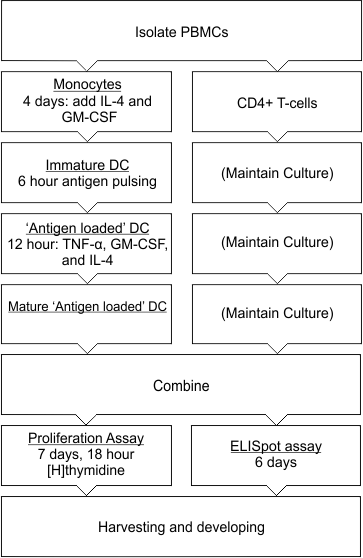
Monitoring T-dependent lymphocyte activation in vitro is an established means for exploring and characterizing the relative immunogenicity potential of foreign proteins. This can be accomplished by co-culturing antigen loaded dendritic cells with T-lymphocytes obtained from peripheral blood mononuclear cells from human or animal donors as illustrated in Figure 1 below. Secretion of two cytokines, interleukin-2 (IL-2) and interferon gamma (IFN-g), from the T-lymphocytes, as well as cellular proliferation, are measured in response to the presentation of an individual test antigen.
Source: Adapted from Jaber & Baker (2007). Assessment of the immunogenicity of different interferon beta-1a formulations using ex vivo T-cell assays. Journal of Pharmaceutical and Biomedical Analysis 13: 1256-1261.
T-cell Activation Endpoint Analyses
- Proliferation
- Bromodeoxyuridine (BrdU) incorporation
- Carboxyfluorescein succinimidyl ester (CFSE)
- Activation Markers (CD69, HLA-DR, IL-25)
- Cytokines
- Immunoassay (single or multiplex)
- Elispot Assay (IFN-y)
References
De Groot A and Moise L (2007). Prediction of immunogenicity for therapeutic proteins: State of the art. Current Opinion of Drug Discovery and Development 10(3):332-340.
Jaber A and Baker M (2007). Assessment of the immunogenicity of different interferon beta-1a formulations using ex vivo T-cell assays. Journal of Pharmaceutical and Biomedical Analysis 43: 1256-1261
Jawa V, Cousens LP, Awwad M, Wakshull E, Kropshofer H and De Groot AS (2013). T-cell dependent immunogenicity of protein therapeutics: Preclinical assessment and mitigation. Clinical Immunology 149: 534-555.